Clinical trials
Clinical trials are sets of tests in medical research that generate information on health interventions (e.g., drugs, diets, diagnostics, devices, therapy protocols).
Clinical trials often involve patients with specific health conditions. In early phases, participants are healthy volunteers who receive financial incentives for their inconvenience. In coordination with a panel of expert investigators, the sponsor decides what to compare the new agent with (one or more existing treatments or a placebo), and what kind of patients might benefit from the medication or device.
During the clinical trial, the investigators: recruit patients with the predetermined characteristics, administer the treatment(s), and collect data on the patients' health for a defined time period. These patients are volunteers and they are not paid for participating in clinical trials.
Clinical trials often involve patients with specific health conditions. In early phases, participants are healthy volunteers who receive financial incentives for their inconvenience. In coordination with a panel of expert investigators, the sponsor decides what to compare the new agent with (one or more existing treatments or a placebo), and what kind of patients might benefit from the medication or device.
During the clinical trial, the investigators: recruit patients with the predetermined characteristics, administer the treatment(s), and collect data on the patients' health for a defined time period. These patients are volunteers and they are not paid for participating in clinical trials.
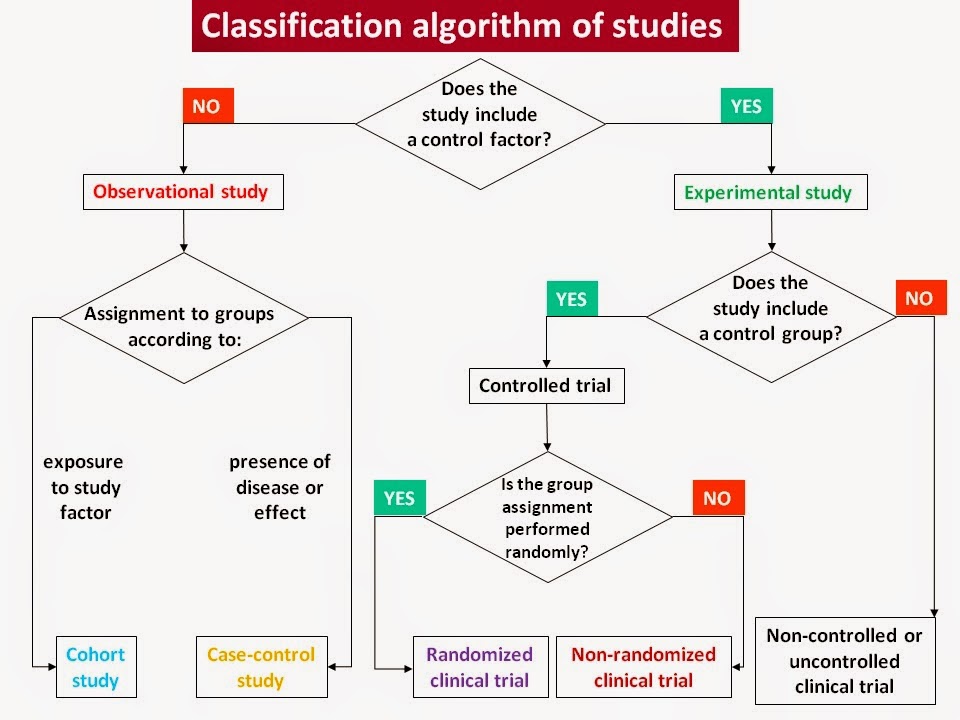
Classification of clinical trials can be done by the way the researchers behave:
- In an observational study, the investigators observe the subjects and measure their outcomes.
- In an interventional study, the investigators give the research subjects a particular treatment (pharmacological, dietary or surgical). Usually, they compare the treated subjects to subjects who receive no treatment or standard treatment. Then the researchers measure how the subjects' health changes.
Taking into account their purpose five different types of trials can be organized:
- Prevention trials look for better ways to prevent disease in people who have never had the disease or to prevent a disease from returning. These approaches may include medicines, vitamins, vaccines, minerals, or lifestyle changes.
- Screening trials test the best way to detect certain diseases or health conditions.
- Diagnostic trials are conducted to find better tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test experimental treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Quality of life trials (supportive care trials) explore ways to improve comfort and the quality of life for individuals with a chronic illness.
- Compassionate use trials provide partially tested, unapproved therapeutics to a small number of patients who have no other realistic options. Usually, this involves a disease for which no effective therapy exists, or a patient who has already attempted and failed all other standard treatments and whose health is so poor, he does not qualify for participation in randomized clinical trials.
The following videos show the importance of randomization and blinding.
The traditional categorization of research identifies two main categories: basic research (also termed fundamental or pure research) and applied research. While basic research often leads to breakthroughs or paradigm-shifts in practice, it usually takes a long time to be applied in any practical context. On the contrary, applied biomedical investigation often represents an incremental improvement to current knowledge rather than a radical breakthrough, however, it has the advantage of exerting a potential direct impact on clinical practice in a relatively short time period. As mentioned by Albani & Prakken in their very comprehensive article published in the 2009 September issue of Nature Medicine, translational medicine encompasses the continuum of activities that extend from the conception of an idea to advanced clinical testing and, ultimately, to the development of a new medical technology or drug. Translational research refers to making findings from basic science useful for practical applications that improve human health and well-being. With a focus on multidisciplinary collaboration, translational research aims to move “from bench to bedside” or from laboratory experiments through clinical trials to point-of-care patient applications. However, translational research is bidirectional and also encompasses “from bedside to bench” approaches.
Compartmentalization within science together with the cultural separation between different scientific fields makes it difficult to establish the multidisciplinary teams that are necessary to be successful in translational research. Additional challenges for translational research arise in the traditional roadblocks encountered in academia, industry and government to successfully move an idea from its conceptual stage to early clinical development and final application. To flourish, translational research requires a knowledge-driven system that establishes a feedback loop to accelerate the translation of data into knowledge whereby the complex and underlying causes and outcomes of disease can be unravelled, to design either effective prevention processes or early detection and personalized treatment strategies. Thus, translational research entails two distinct domains, namely the enterprise of translating knowledge from the basic sciences into the development of new treatments at the same time as translating the findings from clinical trials into everyday practice.
Compartmentalization within science together with the cultural separation between different scientific fields makes it difficult to establish the multidisciplinary teams that are necessary to be successful in translational research. Additional challenges for translational research arise in the traditional roadblocks encountered in academia, industry and government to successfully move an idea from its conceptual stage to early clinical development and final application. To flourish, translational research requires a knowledge-driven system that establishes a feedback loop to accelerate the translation of data into knowledge whereby the complex and underlying causes and outcomes of disease can be unravelled, to design either effective prevention processes or early detection and personalized treatment strategies. Thus, translational research entails two distinct domains, namely the enterprise of translating knowledge from the basic sciences into the development of new treatments at the same time as translating the findings from clinical trials into everyday practice.
- Study the different classification possibilities of research studies together with their main characteristics, advantages and disadvantages.
- This information will enable you to decide which is the most appropriate type of research in each case.
- You need to know and understand what "translational research" means.
Go to ADI to see the assignment of topics for the team work!


No hay comentarios:
Publicar un comentario
Nota: solo los miembros de este blog pueden publicar comentarios.